Computational photochemistry
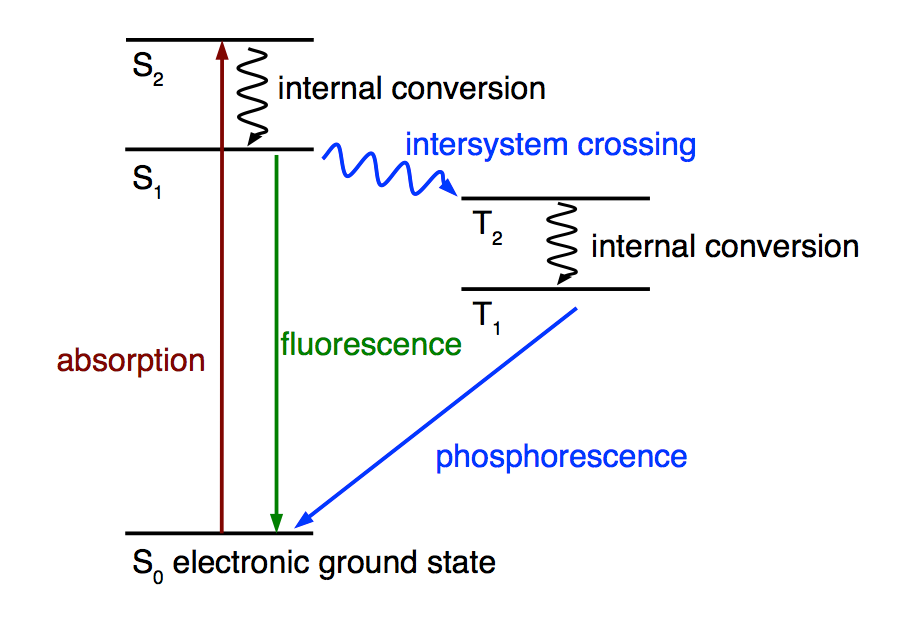
Jablonski diagram, illustrating the electronic states in a molecule and transitions between them.
A variety of processes may be initiated in molecules by the absorption of light. For example, some molecules are fluorescent, some are phosphorescent, some are good photosensitizers. We want to understand why. What makes a molecule strongly fluorescent? Which structural elements are needed to make a good photosensitizer?
A photosensitizer is a molecule which, after absorption of light, produces a chemical change in another molecule. An example for such a change is the transformation of triplet oxygen to singlet oxygen. A prerequisite for a potent photosensitizer is the existence of a long lived triplet excited state and its efficient population via intersystem crossing (ISC). This obviously requires that ISC is faster than competing processes such as fluorescence.
For answering the questions mentioned above computational methods are needed which are able to describe all processes that may occur in a molecule after absorption of a photon. This is still not routine. In the group of Christel Marian, our collaboration partner, a number of efficient quantum mechanical methods have been developed which allow the study of these processes even in relatively large molecules.
Using these methods we recently we have investigated a series of phenothiazinium dyes. These chromophores are widely used for cellular staining, for the decontamination of blood products, as redox biosensors, as fluorescent labels, and as antimicrobials. Some of these photosensitizers have potential applications in photodynamic therapy.
Despite their apparent similarity, the photophysical behavior of these molecules is quite different. While oxonine is strongly fluorescent (fluorescence quantum yield approximately 100%), fluorescence in thionine is much weaker and thionine is a photosensitizer with a singlet oxygen quantum yield of about 0.5. We have been able to reproduce and explain this trend and make the prediction that selenine may be an even better photosensitizer than thionine.